Clinical trial Research environment in Vietnam
There is continuous growth in the scientific base and capabilities in Vietnam, encouraged by local authorities, given the growing contribution of clinical research to the local economy.
Trials are supported by Clinical Research Units (CRUs) in many large hospitals and study coordinators.
Efficient regulatory and ethics committee processes
The trend among local ethics commmittee and regulatory authorities in Vietnam is towards reduction of regulatory approval timelines. Vietnam Ministry of Health continues to provide clear instructions on their processes and requirements for documentation.
Some main activities by Vietnam MOH to improve clinical trial research environment locally:
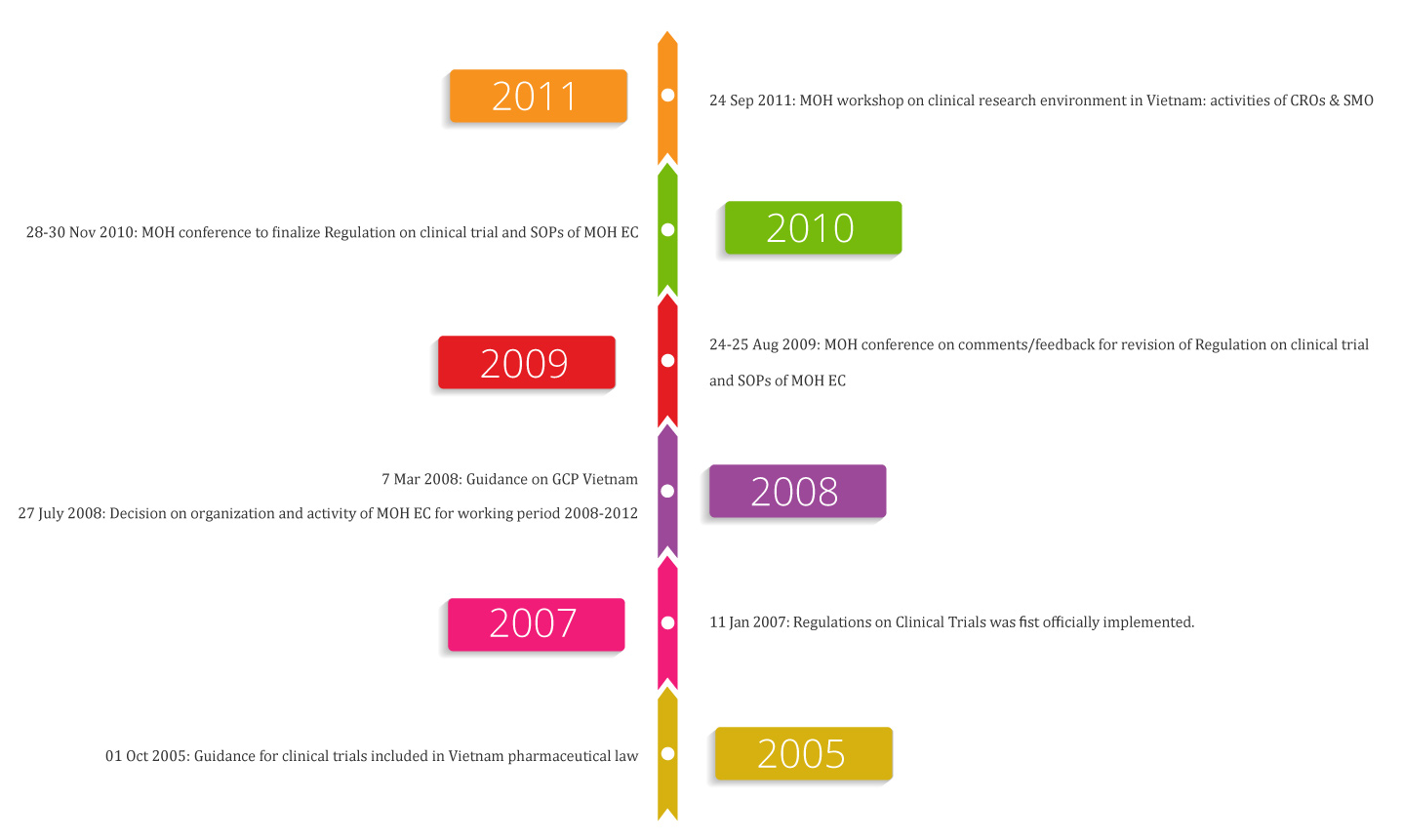

24 Sep 2011: MOH workshop on clinical research environment in Vietnam: activities of CROs & SMO

28-30 Nov 2010: MOH conference to finalize Regulation on clinical trial research and SOPs of MOH EC

24-25 Aug 2009: MOH conference on comments/feedback for revision of Regulation on clinical trial research and SOPs of MOH EC

7 Mar 2008: Guidance on GCP Vietnam
27 July 2008: Decision on organization and activity of MOH EC for working period 2008-2012

11 Jan 2007: Regulations on Clinical Trials was fist officially implemented

01 Oct 2005: Guidance for clinical trials included in Vietnam pharmaceutical law
Investigational sites and investigators’ knowledge and experience
More and more hospitals/institutions and investigators in Vietnam are involved in GCP clinical trial research, global as well as local, therefore, their knowledge and experience have recently developed and helped to bring more trials research to Vietnam. According to 2011 update from Department of Science and Training, Vietnam MOH, the number of investigators participated instructor-led training and received GCP certificates from MOH is approximately 500.
Faster patient recruitment and good retention
Vietnam can offer large patient pool, with US/EU treatment guidelines are followed in the treatment of disease in main therapeutic areas.
Typically, doctor-patient relationship is strong in Vietnam, thus securing good patient retention by sites.
There are still fewer competiting trials compared to the other South East Asian countries.
Cost benefits
Including investigator sites in Vietnam can help reduce the overall drug development timelines, with a higher number of patients in fewer sites enable faster patient recruitment.
Cost for conducting clinical trials in Vietnam is still favorable when compared to other countries.
Logistics
There are strict customs regulations within the country to comply with.
Strategies to deal with the specificities of logistics in Vietnam include building local knowledge of regulations and import procedures related to clinical trials research, as well as designing an adequate distribution approach, together with third parties.

